Clean Technology for the Treatment and Modelling of Acid Mine Drainage Effluent
Padmaja Megham1 *
Corresponding author Email: padmajamegham@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.15.Special-Issue1.08
Copy the following to cite this article:
Megham P. Clean Technology for the Treatment and Modelling of Acid Mine Drainage Effluent. Curr World Environ 2020; Special Issue (Sustainable Mining). DOI:http://dx.doi.org/10.12944/CWE.15.Special-Issue1.08
Copy the following to cite this URL:
Megham P. Clean Technology for the Treatment and Modelling of Acid Mine Drainage Effluent. Curr World Environ 2020; Special Issue (Sustainable Mining). Available from: https://bit.ly/2NGYD8Q
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 14-05-2020 |
|---|---|
| Accepted: | 26-06-2020 |
| Reviewed by: | 
 Debasish Pandi
Debasish Pandi
|
| Second Review by: |

 Anup Choudhary
Anup Choudhary
|
| Final Approval by: | Prof BB Dhar |
Introduction
The economic development of any country mainly depends significantly on the mining industry.1 Majorly, the mining operations generate vast quantities of wastewater as the result of AMD.2, 3 The significant effect of the mining is the degradation of natural resources.4 The amount of water used in the mining industry is huge and generally higher than what is predicted due to the mining processes involved. Furthermore, the effluent from slag washing has high acidity (pH<4) and is polluted with elevated levels of heavy metals.5-7
The long-term effects due to metal-ions (Cd, Ni, Cu, Hg, Cr, Zn etc.) even in mild concentrations are bio-accumulation and heavy metal poisoning.8
The conventional Physico-chemical processes were employed in the treatment of AMD like coagulation-flocculation, sedimentation, and filtration. Researchers have been researching for easy, clean, and low-cost viable treatment options since the last decade.9 In this context, adsorption technology would be a competent solution in the treatment of AMD effluent.10-12
The tedious batch experiments conducted for adsorption study can be optimized using statistical tool Response Surface Methodology (RSM).13, 14 Recently, a study based on the tool optimized the following: -
- Adsorption of Fluoride wastewater using modified soil as adsorbent using central composite design RSM.15
- Adsorption of cationic metals Copper and Zinc on silica and optimized batch tests using RSM.16
- Dye sorption from aqueous solutions17 by using Nanocomposites as adsorbents.
On the other hand, artificial Neural Networks (ANNs), tools based on Artificial intelligence that are used by many researchers to simplify the mathematical calculations. ANNs were used for:-
- Optimization and modeling the sorption of Copper and Lead using rice straw as adsorbent.18
- Biosorption process using various agricultural wastes in treating the metal-polluted waters.19
A combination of RSM and ANNs was used by many researchers for process optimization, statistical modeling in the adsorption process.20
The current study illustrates the batch adsorption onto composite vegetable waste carbon (CVWC) as a low-cost treatment option for AMD effluent.21, 22 There is a two-fold advantage, one is the reduction of vegetable wastes which end up in the open dumps and economic development by the utilization of the wastes for treating wastes. The process optimization was carried out using RSM and modeling was done by Feed Forward Back Propagation Neural network. The regression models in both RSM and ANN were compared.
Materials and Methods
Adsorbate and Adsorbent Preparation
The real time AMD effluent was gathered from Iron ore mines located at Bayyaram, Telangana, India. According to USEPA, Cadmium, Copper, Nickel, Arsenic, Chromium, Lead, Zinc, and Mercury are the most toxic heavy metals discharged into the water environment.23 The Characteristics of AMD effluent and US EPA effluent standards are shown in Table. 1. Three heavy metals Cadmium, Zinc, and iron (highlighted in Table. 1) are deviating from the US EPA standards, and hence those are considered for the study.
Table 1: AMD Effluent Characteristics and corresponding USEPA effluent disposal standards
|
Parameter |
Concentration (mg/L) |
US EPA standards (mg/L) |
|
Cd |
12.34 |
2.0 |
|
Cu |
1.54 |
3.0 |
|
Zn |
96.5 |
5.0 |
|
Cr |
0.05 |
0.1 |
|
Co |
0.023 |
0.05 |
|
Ni |
0.13 |
3.0 |
|
Fe |
146.75 |
5.0 |
|
Pb |
0.013 |
0.1 |
|
Hg |
0.0003 |
0.01 |
A 100% dilution ratio was considered for the laboratory scale batch study. The Composite Vegetable Waste (CVW) was used as adsorbent collected from local vegetable markets. The CVW was then dried sufficiently under sunlight, washed with plenty of water to remove any grit or sand. Again, the mass was air-dried, followed by oven drying at 110 ÌŠ C overnight. The recovered mass was roughly ground after cooling and was carbonized at 450 ÌŠ C for 2 hours. The so obtained product was Composite Vegetable Waste Carbon (CVWC).
Experimental
A laboratory-scale batch study was carried out for the elimination of Metal-ions from real-time AMD effluent after subsequent dilution (sample volume (v) 100 mL).24, 25 The effect of process parameters for instance pH, adsorbent dose (M), the contact time was reviewed. The ranges selected for the parameters are pH (2 to 9), adsorbent dose (1-10 mg), and contact time (0.5 to 3 hours). The pre concentration (Co) and post concentration(Ce) of the metals were obtained by spectrophotometry, and their % removal (%R) and adsorption capacity qe(mg/g) was analysed as given in Equation (1) ad (2).
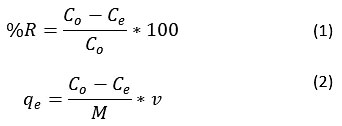
RSM Optimization
The 2-factorial 3-level central composite design in RSM used to optimize process factors. The effect of each factor, as well as the interaction, was studied. The optimum values of all the process variables coded values (-1, 0, +1) were obtained by replicating the experiments six times at center points. A quadratic expression is used in the optimization process as shown in Equation (3)
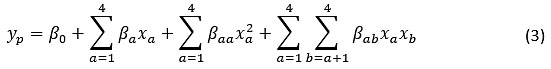
Where, yp is the response, i.e. the % removal and adsorption capacity, β0 (constant), βa(linear), βaa(quadratic) and βab (interaction) are coefficients, respectively. xa (a= to 4) is the independent factor affecting the response. The coefficient of determination (R2) was obtained from the ANOVA method in RSM using Design Expert® software.
ANNs Modelling
Matlab® environment was employed for modeling the experimental feed results using Feed Forward back propagation neural network. Of many forms of algorithms, the Levenberg-Marquardt (LM) was employed as it was more appropriate and considered for modeling. A total of 20 samples were considered 14 for training, three each for validation and testing. The performance of batch tests was analyzed using the ANNs model, and regression analysis compared to RSM.
Results and discussion
Experimental
The experimental details given in Table 2 highlights the effect of pH, adsorbent dose, contact time on multi-metal removal. The Optimum pH for Cd, Zn, and Fe was 5.5. The adsorbent dose was 5.5 for Fe and 9 for Cd and 10 for Zn. The optimum contact time was at 1.75 Hrs for all the metals. Fig. 1 (a), (b), (c) explains the effect of selected factors on the removal (%R).
CCD Design
Tests were performed on factors and their effect on the sorption onto CVWC. A 3-level full factorial central composite face-centered design and 25 runs with 3 (-1, 0, +1) center points was used to realize the effects of many process parameters upon sorption of the three metals Cadmium, Zinc and Iron.27,28 Tests (20) were conducted randomly as per the selection by factorial design, as shown in Table 2. The effect of test factors like pH, Adsorbent dose, and contact time were studied upon the % removal using Origin® Pro Software.
Final Equation in Terms of Coded Factors
%R (Cd) = 84.70 + 8.16 A + 0.9210 B + 7.39 C + 6.45 AB + 5.44 AC + 11.93 BC - 18.78 A2 + 0.2155 B2 - 11.96 C2
%R (Zn) = 84.97-2.40 A + 6.91 B + 2.37 C + 0.0775 AB + 4.59 AC + 8.82 BC - 12.89 A2 + 0.2000 B2 - 1.08 C2
%R (Fe) = 85.78 + 3.13 A + 14.15 B + 0.2950 C - 3.14 AB + 0.1825 AC + 11.43 BC -22.62 A2 - 2.67 B2 + 6.93 C2
The predictions about each factor and their response arrived from the equation in relation to the coded factors. The coded levels +1 and -1 denotes high to low with reference to coded factors.
ANNs Modelling
Back propagation Neural Network was used for modeling of experimental data onto CVWC.25, 26 The network has 3 input and output neurons, the hidden and output layer has 10 and 3 neurons, respectively.
Comparison of RSM and ANNs models
The regression analysis for tedious problems can be analyzed using RSM and ANNs. These models were employed to investigate the adsorption of AMD wastes onto CVWC.26-28 The statistical analysis based on RMSE and R2 were calculated based on the following equations for RSM and ANNs presented in Table 3.
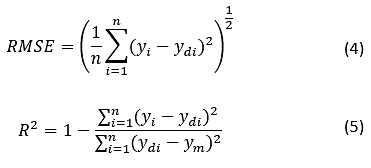
Where n refers to the number of points, yi being the predicted (RSM & ANNs) value, ydi is the actual (experimental) value, and ym is the mean of the actual values.
The RMSE for RSM and ANNs were reported to be 0.346 and 1.258 respectively; this shows that the values of ANNs deviated compared to RSM. R2 (coefficient of correlation) for RSM and ANNs were obtained to be 0.89 and 0.96, respectively. The regression analysis gives an idea about how well data fits the model. Fig. 2 (a), (b), (c) convey the comparison for removal (%R) for experimental, RSM, and ANNs predicted values. No significant deviation from the experimental data was found.
Though RSM and ANNs models fit well to experimental data, comparatively, the ANNs model was more dominant than RSM. However, RSM is advantageous over ANNs in depicting the relationships between various operational factors in terms of responses. However, the major drawback of RSM is that it presumes only a quadratic form of non-linear correlation. However, ANNs have an inbuilt system that can naturally encapsulate most of the non-linearity, in contrast to RSM.
Table 2: Effect of factors on % R (Experimental, RSM and ANNs) for Cd, Zn and Fe
|
Run |
pH (A) |
Ads. Dose (mg) (B) |
Contact Time (Hrs) (C) |
%R (Cd) |
%R (Zn) |
%R (Fe) |
||||||
|
Exp |
RSM |
ANNs |
Exp |
RSM |
ANNs |
Exp |
RSM |
ANNs |
||||
|
1 |
9 |
5.5 |
1.75 |
86.45 |
85.89 |
85.77 |
78.45 |
79.21 |
78.76 |
67.45 |
66.89 |
67.21 |
|
2 |
5.5 |
1 |
1.75 |
54.33 |
54.76 |
53.89 |
67.33 |
67.45 |
67.19 |
55.45 |
55.22 |
55.33 |
|
3 |
5.5 |
10 |
1.75 |
93.56 |
92.80 |
93.06 |
93.45 |
93.90 |
92.32 |
90.35 |
90.56 |
90.76 |
|
4 |
5.5 |
5.5 |
1.75 |
91.76 |
90.99 |
90.96 |
85.43 |
85.11 |
85.41 |
91.56 |
91.89 |
91.77 |
|
5 |
5.5 |
5.5 |
1.75 |
92.33 |
92.78 |
92.58 |
86.43 |
86.04 |
86.07 |
91.41 |
91.33 |
91.34 |
|
6 |
2 |
1 |
0.5 |
72.16 |
72.57 |
72.79 |
81.32 |
80.80 |
82.10 |
63.22 |
63.15 |
63.25 |
|
7 |
9 |
1 |
0.5 |
68.34 |
68.65 |
69.05 |
64.66 |
64.34 |
64.32 |
75.38 |
74.45 |
75.87 |
|
8 |
9 |
10 |
0.5 |
34.56 |
32.98 |
34.06 |
53.65 |
53.21 |
53.90 |
67.23 |
67.78 |
67.16 |
|
9 |
2 |
10 |
0.5 |
43.22 |
43.64 |
43.48 |
78.54 |
78.09 |
78.21 |
78.99 |
79.49 |
78.54 |
|
10 |
5.5 |
5.5 |
3 |
89.32 |
90.02 |
89.37 |
79.56 |
80.05 |
79.56 |
88.9 |
88.54 |
89.43 |
|
11 |
5.5 |
5.5 |
1.75 |
92.67 |
92.21 |
91.59 |
86.77 |
86.22 |
86.12 |
94.89 |
95.12 |
93.78 |
|
12 |
9 |
1 |
3 |
44.74 |
43.90 |
43.99 |
57.63 |
57.32 |
57.36 |
44.76 |
44.24 |
44.23 |
|
13 |
5.5 |
5.5 |
1.75 |
90.56 |
90.78 |
91.21 |
89.51 |
90.03 |
88.98 |
91.31 |
91.53 |
91.81 |
|
14 |
2 |
10 |
3 |
45.56 |
45.34 |
45.44 |
88.41 |
88.77 |
88.11 |
53.34 |
53.61 |
53.62 |
|
15 |
5.5 |
5.5 |
1.75 |
91.34 |
91.86 |
91.41 |
90.48 |
90.66 |
90.32 |
89.77 |
89.05 |
89.09 |
|
16 |
5.5 |
5.5 |
0.5 |
34.22 |
33.81 |
34.06 |
78.65 |
77.79 |
78.16 |
76.09 |
77.31 |
75.74 |
|
17 |
9 |
10 |
3 |
89.33 |
89.01 |
88.21 |
90.44 |
90.67 |
90.17 |
93.65 |
93.67 |
93.96 |
|
18 |
5.5 |
5.5 |
1.75 |
93.43 |
93.87 |
93.53 |
90.3 |
90.21 |
90.87 |
96.57 |
96.34 |
96.32 |
|
19 |
2 |
1 |
3 |
57.45 |
57.71 |
56.18 |
64.45 |
64.87 |
64.34 |
43.21 |
43.45 |
43.34 |
|
20 |
9 |
1 |
1.75 |
23.45 |
24.78 |
22.63 |
56.15 |
56.64 |
56.35 |
38.45 |
39.17 |
39.17 |
*A, B, C are coded factors for pH, adsorbent Dose and Contact time
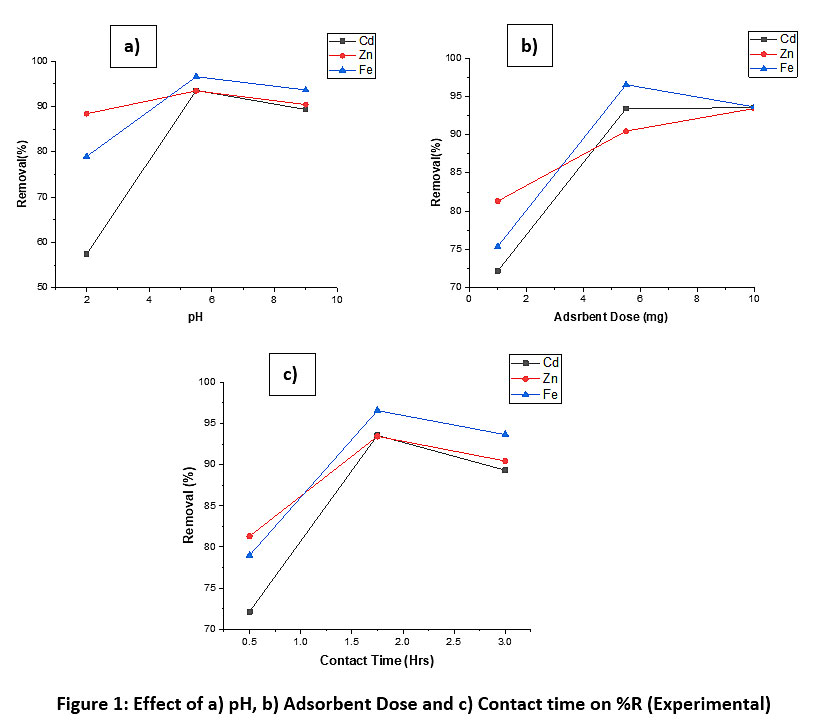 |
Figure 1: Effect of a) pH, b) Adsorbent Dose and c) Contact time on %R (Experimental) Click here to view Figure |
 |
Figure 2: Experimental %R versus predicted (RSM & ANNs) for a) Cd b) Zn and c) Fe Click here to view Figure |
Conclusion
The study supports that combined vegetable waste carbon (CVWC) is effective in the Treatment of AMD effluent in an eco-friendly manner. The laboratory-scale batch study was successful in the elimination of the three metals, i.e. Cadmium, Zinc, and Iron from AMD effluent. The experimental data revealed the highest removal was observed for Iron at an optimum pH and the adsorbent dose of 5.5 and at a contact time of 1.75 hours, respectively. RSM and ANNs models were employed to forecast the adsorption efficiency of the metals from wastewater onto CVWC. RMSE and R2 were used to predict the working of RSM and ANNs models. The plots with experimental versus predicted data revealed a good correlation with the experimental data.
References
- Ericsson, Magnus, and Olof Löf. Mining’s contribution to national economies between 1996 and 2016. Mineral Economics. 2019:32(2):223-250.
- Abinandan, Sudharsanam, Suresh R. Subashchandrabose, Kadiyala Venkateswarlu, and Mallavarapu Megharaj. Microalgae–bacteria biofilms: a sustainable synergistic approach in remediation of acid mine drainage. Applied microbiology and biotechnology. 2018;102(3):1131-1144.
- Ballester, Antonio, Laura Castro, Maria Clara Costa, Jorge Carlier, Manuel García-Roig, Patricia Pérez-Galende, Angela Alvarez, Caroline Bertagnolli, and Eric Guibal. Design of remediation pilot plants for the treatment of industrial metal-bearing effluents (BIOMETAL DEMO project): Lab tests. Hydrometallurgy. 2017;168:103-115.
- Ergüler, Güzide Kalyoncu. Investigation the applicability of eggshell for the treatment of a contaminated mining site. Minerals Engineering. 2015;76:10-19.
- Zhang, Ting, Zhihong Tu, Guining Lu, Xingchun Duan, Xiaoyun Yi, Chuling Guo, and Zhi Dang. Removal of heavy metals from acid mine drainage using chicken eggshells in column mode. Journal of environmental management. 2017;188:1-8.
- de Paiva Magalhães, Danielly, Mônica Regina da Costa Marques, Darcilio Fernandes Baptista, and Daniel Forsin Buss. Metal bioavailability and toxicity in freshwaters. Environmental chemistry letters. 2015;13(1):69-87.
- Abdolali, Atefeh, Huu Hao Ngo, Wenshan Guo, John L. Zhou, Jian Zhang, Shuang Liang, Soon W. Chang, Dinh Duc Nguyen, and Yi Liu. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresource technology. 2017; 229:78-8
- Brahmi, Khaled, Wided Bouguerra, Soumaya Harbi, Elimame Elaloui, Mouna Loungou, and Béchir Hamrouni. Treatment of heavy metal polluted industrial wastewater by a new water treatment process: ballasted electroflocculation. Journal of hazardous materials. 2018;344:968-980.
- Poo, Kyung-Min, Eun-Bi Son, Jae-Soo Chang, Xianghao Ren, Yun-Jung Choi, and Kyu-Jung Chae. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. Journal of environmental management. 2018;206:364-372.
- Vijayaraghavan, K., and R. Balasubramanian. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. Journal of environmental management. 2015;160:283-296.
- Rout, Prangya Ranjan, Rajesh Roshan Dash, and Puspendu Bhunia. Nutrient removal from binary aqueous phase by dolochar: highlighting optimization, single and binary adsorption isotherms and nutrient release. Process Safety and Environmental Protection. 2016;100:91-107.
- Åžahan, Tekin. Application of RSM for Pb (II) and Cu (II) adsorption by bentonite enriched with SH groups and a binary system study. Journal of Water Process Engineering. 2019;31:100867.
- Roy, Swapnila, Papita Das, Shubhalakshmi Sengupta, and Suvendu Manna. Calcium impregnated activated charcoal: Optimization and efficiency for the treatment of fluoride containing solution in batch and fixed bed reactor. Process Safety and Environmental Protection. 2017;109:18-29.
- Mousavi, Seyyed Javad, Mehdi Parvini, and Mohsen Ghorbani. Adsorption of heavy metals (Cu2+ and Zn2+) on novel bifunctional ordered mesoporous silica: Optimization by response surface methodology. Journal of the Taiwan Institute of Chemical Engineers. 2018;84:123-141.
- Mahmoodi, Niyaz Mohammad, Mohsen Taghizadeh, and Ali Taghizadeh. Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling. Journal of Molecular Liquids. 2019; 277:310-322.
- Khandanlou, Roshanak, Hamid Reza Fard Masoumi, Mansor B. Ahmad, Kamyar Shameli, Mahiran Basri, and Katayoon Kalantari. Enhancement of heavy metals sorption via nanocomposites of rice straw and Fe3O4 nanoparticles using artificial neural network (ANN). Ecological Engineering. 2016; 91:249-256.
- Liu, Zhi-Wei, Fang-Nan Liang, and You-Zhi Liu. Artificial neural network modeling of biosorption process using agricultural wastes in a rotating packed bed. Applied Thermal Engineering. 2018;140:95-101.
- Dil, Ebrahim Alipanahpour, Mehrorang Ghaedi, Arash Asfaram, Fatemeh Mehrabi, Ali Akbar Bazrafshan, and Abdol Mohammad Ghaedi. Trace determination of safranin O dye using ultrasound assisted dispersive solid-phase micro extraction: Artificial neural network-genetic algorithm and response surface methodology. Ultrasonics sonochemistry. 2016; 33:129-140.
- Dehghani, Mohammad Hadi, Kaan Yetilmezsoy, Mehdi Salari, Zoha Heidarinejad, Mahmood Yousefi, and Mika Sillanpää. Adsorptive removal of cobalt (II) from aqueous solutions using multi-walled carbon nanotubes and γ-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. Journal of Molecular Liquids. 2020; 299:112-154.
- Lata, Sneh, and S. R. Samadder. Removal of arsenic from water using nano adsorbents and challenges: a review. Journal of environmental management. 2016; 166:387-406.
- Sabela, Myalowenkosi I., et al. Removal of copper (II) from wastewater using green vegetable waste derived activated carbon: An approach to equilibrium and kinetic study. Arabian Journal of Chemistry. 2019: 12(8):4331-4339.
- Aziz, El Kassimi, et al. Adsorptive removal of anionic dye from aqueous solutions using powdered and calcined vegetables wastes as low-cost adsorbent. Arab Journal of Basic and Applied Sciences. 2018:25(3): 93-102.
- Åžahan, Tekin, Hasan Ceylan, and Nahit AktaÅŸ. Optimization of biosorption of Zn (II) ions from aqueous solutions with low-cost biomass Trametes versicolor and the evaluation of kinetic and thermodynamic parameters. Desalination and Water Treatment. 2016;57(26):12156-12167.
- Sabela, Myalowenkosi I., et al. Removal of copper (II) from wastewater using green vegetable waste derived activated carbon: An approach to equilibrium and kinetic study. Arabian Journal of Chemistry. 2019:12(8): 4331-4339.
- Kooh, Muhammad Raziq Rahimi, et al. Batch adsorption studies of the removal of methyl violet 2B by soya bean waste: isotherm, kinetics and artificial neural network modelling. Environmental Earth Sciences. 2016:75(9):783.
- Dehghani, Mohammad Hadi, et al. Adsorptive removal of cobalt (II) from aqueous solutions using multi-walled carbon nanotubes and γ-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. Journal of Molecular Liquids. 2020:299:112-154.
- Zin, Khairunnisa’Mohd, et al. Microbial Decolorization of Triazo Dye, Direct Blue 71: An Optimization Approach Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN). BioMed Research International. 2020:2020.
- Jaskulak, Marta, Anna Grobelak, and Franck Vandenbulcke. Modeling and optimizing the removal of cadmium by Sinapis alba L. from contaminated soil via Response Surface Methodology and Artificial Neural Networks during assisted phytoremediation with sewage sludge. International Journal of Phytoremediation. 2020:1-10.






